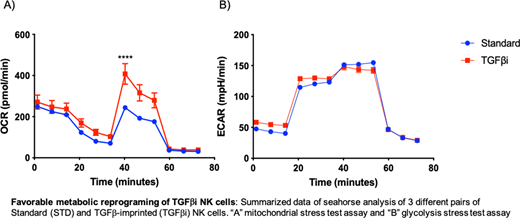
Transforming growth factor-β (TGF-β) plays an essential role in regulating immune responses through its immunossupressive effect on adaptive and innate immune cells. In addition, we and others have shown that alterations in cell-intrinsic metabolism profiles can significantly impact the function of immune cells. Previously, our group demonstrated that TGFβ-imprinting (TGFβi) decreases NK cell sensitivity to TGFβ through SMAD3 suppression and enhances a pro-inflammatory phenotype with hypersecretion of IFN-γ, TNF-α, and GM-CSF, which appeared to be mediated by an epigenetic mechanism. To evaluate this process, we conducted RNA-seq and ATAC-seq to evaluate the impact of chromosomal remodeling on gene expression. In addition to confirming low SMAD3 transcription, this data showed that TGFβi NK cells have decreased CD38 expression, which we confirmed at the protein level. Since CD38 is an ectoenzyme that regulates nicotinamide adenine dinucleotide (NAD+), a critical component of OXPHOS in both T and NK cells, we examined the effect of TGFβi on NK cell metabolism. TGFβi primary NK cells were generated from peripheral blood of healthy donors as previously described, and using a mitochondrial stress test assay, we observed higher oxygen consumption rates (OCR). However, in the glycolysis stress test assay we observed comparable extracellular acidification rates (ECAR) between Standard expanded and TGFbi NK cells, resulting in higher OCR/ECAR ratios in TGFbi NK cells. Since TGFβ has been shown to induce fragmented mitochondria, and inhibition of mitochondrial fragmentation improved mitochondrial metabolism, we evaluated subcellular structure of TGFβi NK cellsby transmission electron microscopy (TEM). We found no difference in mitochondrial fragmentation between STD and TGFβi NK cells. Together, these results show that TGFβi induces epigenetic reprograming of NK cells that results in increased OXPHOS through CD38 suppression. Moreover, the decreases CD38 expression in TGFβi NK cells could be a promising non-genetic alternative to generating CD38-negative NK cells to avoid fratricide in combination with DARA
Lee:Kiadis Pharma Netherlands B.V: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal